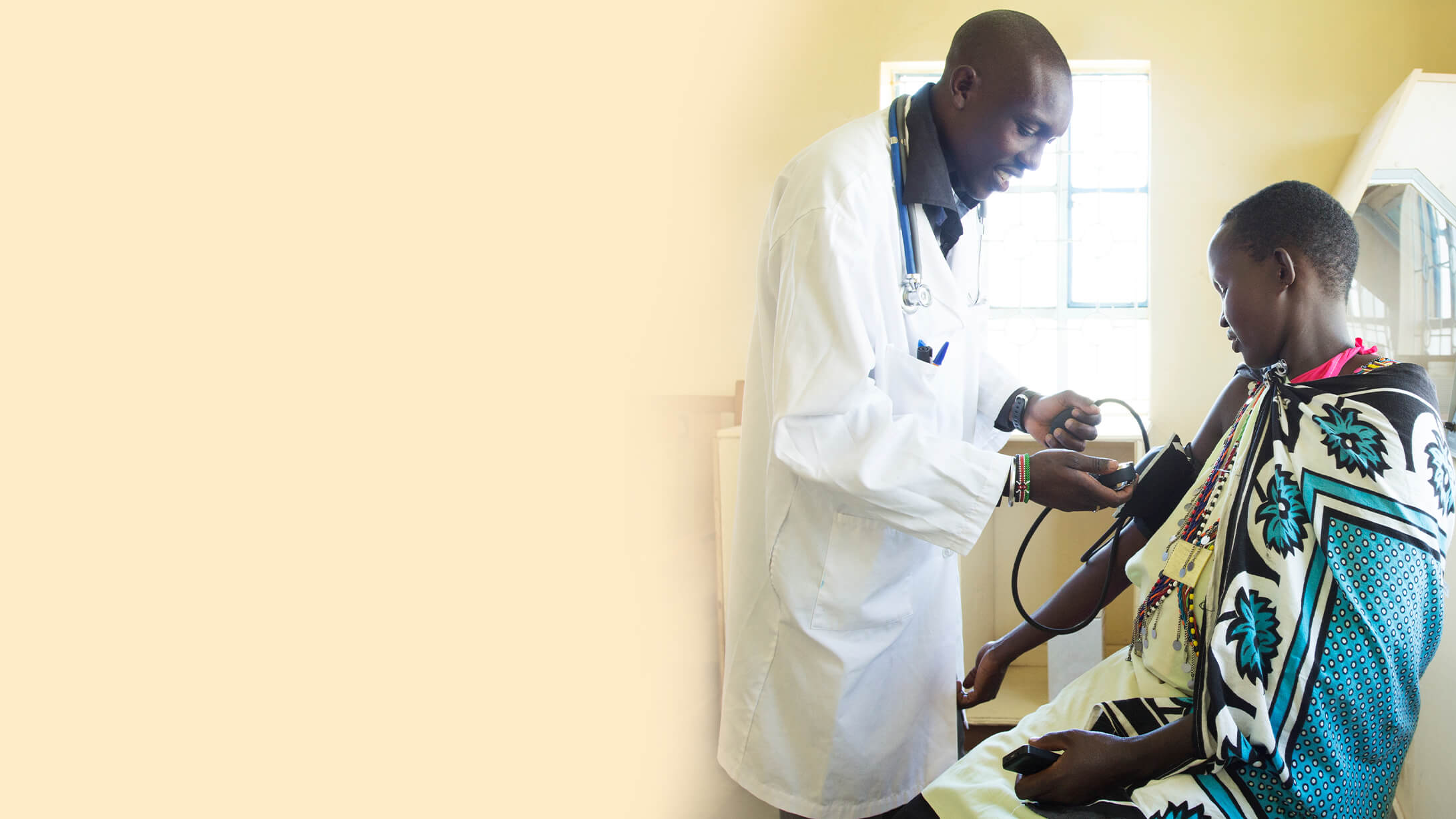
Enabling Global Health Solutions
Cepheid’s Global Access Program centers on the belief that everyone, everywhere should have access to high quality diagnostic tests. Together with our partners, Cepheid brings rapid molecular testing to communities in low- and middle-income countries, improving patient outcomes and trailblazing standards of care.
GeneXpert Solutions are a Critical Tool in the Global Fight to Eliminate TB
More people need access to accurate diagnostics — especially for critical medical conditions like HIV and tuberculosis. That’s why for more than a decade, we have worked to improve healthcare outcomes through expanded access to reliable and fast molecular diagnostic testing solutions. This is a key component of our mission.
In 2010, we ushered in a new era of fast and accurate tuberculosis testing, transforming the outcomes for millions impacted by this disease. Since then, we have continued to work with partners to serve the global community amid ongoing public health emergencies and challenges, including solutions for Anthrax, Ebola, H1N1, SARS, COVID-19, drug-resistant TB testing, and more.
We serve the global community through:
- Innovation: We are dedicated to continuously improving everything we do. Unique to biologic solutions, we address mutations and genetic drift with ongoing investments and innovation.
- Partnerships: We recognize that we cannot fulfill our mission alone. Our ability to deliver results relies on partnerships with policymakers, donors, governments, NGOs, and frontline health workers.
- Proximity: We recognize we must have tests available when and where they are needed most. Therefore, we continue to bring our manufacturing capabilities closer to the markets we serve.
Diagnosis is the critical first step in the path to health, linking patients to care, enabling appropriate treatment, and increasing the likelihood of fast recovery. Cepheid’s Global Access Program was established in 2011 to provide equitable access to diagnostic solutions globally through continuous solution innovation, holistic implementation of these solutions, cross-sector partnerships, and advocacy.
"The diagnostic gap (i.e., the proportion of the population with the condition who remain undiagnosed) is, at 35-62%, the single largest gap in the care pathway (the cascade of care comprising screening, diagnosis, treatment, and cure or successful management)."
Program Updates
Cepheid Supports Global Efforts to Win Against TB
Innovations to Meet the Challenges of Drug Resistance TB in Low-Resource Settings
TBPPM Webinar | Leveraging GeneXpert for Tuberculosis and SARS-CoV-2 Testing
MTB EOL Letter
How We Work
We embed ourselves in the communities we serve to understand their needs, challenges, and preferences and translate these insights into patient-centric solutions: molecular diagnostic systems, tests, and service and support programs. Diseases are not static, so we continuously challenge ourselves to improve existing offerings and to anticipate what the world will need five to ten years from now.
Pushing the envelope for TB diagnostics:
With every launch of a new TB test, Cepheid and its partners have redefined how TB is diagnosed and managed. Since 2006, Cepheid has collaborated with Rutgers University, Foundation for Innovative New Diagnostics (FIND), and National Institute of Allergy and Infectious Disease (NIAID) to harness academic discoveries and scale them for real-world use. The Xpert MTB/RIF* test launched in 2010 with funding support from the Bill & Melinda Gates Foundation. It was a first-of-its-kind molecular test, detecting TB and rifampicin resistance simultaneously. In 2017, Xpert MTB/RIF Ultra* improved on the original test by using detection technology that increased analytical sensitivity by 10-fold and improved detection of rifampicin-related mutations. Xpert MTB/XDR* launched in 2020 using new multiplexing technology to detect resistance to six first- and second-line drugs used for TB treatment.
Instruments and tests alone are not enough, so we strive to provide an ecosystem of awareness and educational initiatives, service, support, and delivery models to strengthen national diagnostic infrastructure.
Accelerating access to Xpert MTB/RIF Ultra:*
Starting in 2017, Cepheid worked with South Africa’s National Health Laboratory System (NHLS) to accelerate access to the new Xpert MTB/RIF Ultra* test. Cepheid’s local Field Application Specialists partnered with the NHLS’ National Priority Programs (NPP) team to make software upgrades required for the test across the instrument network, ensure data transmission into laboratory information systems (LIS) aligned with laboratory requirements, and conduct trainings. The trainings included “train the trainer” sessions for NPP staff, as well as lab employees. In total, Cepheid and NPP employees conducted 70 trainings for more than 200 labs, health centers, and mobile clinics.
We engage directly with donors, policymakers, governments, NGOs, communities, and patients to ensure our work supports global, national, and local health system and disease elimination goals.
Responding to the West African Ebola outbreak:
When the 2014-2016 Ebola outbreak began in West Africa, lack of rapid accurate diagnostics made it difficult to track the outbreak and implement community health measures. Shortly after the World Health Organization (WHO) declared a Public Health Emergency, Cepheid began development of the Xpert Ebola test with a grant from the Paul G. Allen Family Foundation and the Bill & Melinda Gates Foundation. The first Xpert Ebola tests reached health facilities in June 2015, leveraging the existing GeneXpert systems in Sierra Leone, Guinea, and Liberia. Emergency response teams in the field deployed Xpert Ebola for surveillance and diagnosis, establishing detection centers near zones of transmission to prevent further spread.
We speak up for policies and public health initiatives that increase access to molecular testing and reduce health disparities.
Bringing private sector perspective to global disease elimination goals:
Cepheid is a member of the Global Fund and Stop TB Partnership Private Sector Constituencies. In these forums, Cepheid advocates for inclusion of high-quality diagnostics and patient-centered care in global disease elimination plans. The Stop TB Partnership Private Sector Constituency has released white papers on topics including connectivity, sustainable investment, and resilient health systems.
Program History

2006
Cepheid places first GeneXpert® systems
WHO launches Stop TB Strategy on World TB Day

Cepheid, FIND, University of Medicine and Dentistry of New Jersey (Rutgers University), and NIAID collaborate to develop Xpert® MTB/RIF* for TB with a grant from the Bill & Melinda Gates Foundation

2009 - 2010
Xpert MTB/RIF* launches and gets WHO endorsement


2011
Cepheid officially establishes Global Access Program
In response to high rates of TB-HIV co-infection in Global Access countries, Cepheid and FIND partner on development of Xpert HIV-1viral load* (VL) test.

2014
Xpert® HPV* and Xpert HIV-1 VL* tests launch

2015
Cepheid develops and launches Xpert® Ebola test to respond to West African outbreak with funding support from the Paul G. Allen and Bill & Melinda Gates Foundations

Xpert® HIV-1 Qual* and HCV VL* tests launch
2016
WHO prequalifies Xpert HIV-1 Qual*


2017
WHO prequalifies Xpert HCV VL*, Xpert HIV-1 VL*, and Xpert HPV*; Xpert® MTB/RIF Ultra* launches and gets WHO endorsement


2020
Xpert® MTB/XDR* launches to detect multidrug-resistant TB with new 10-color multiplexing technology

Xpert® Xpress SARS-CoV-2^ launches
Global Access Program today
- GeneXpert systems available in multiple modular configuration options
- 13 tests available under Global Access Program, 7 of which are endorsed or pre-qualified by WHO
To request information on our AccessCare Program please complete the following form:
Contact Us
- For media inquiries, contact Darwa.Peterson@cepheid.com.
- For product, partnership, or general inquiries contact ordersinternational@cepheid.com
* CE-IVD. In Vitro Diagnostic Medical Device. May not be available in all countries. Not available in the United States.
^ For use under an Emergency Use Authorization in the United States
IVD. In Vitro Diagnostic Medical Device. May not be available in all countries




