5m Read
COMMUNITY AND GLOBAL HEALTH
Expert Perspective
Accelerating Hepatitis C Elimination in the United States
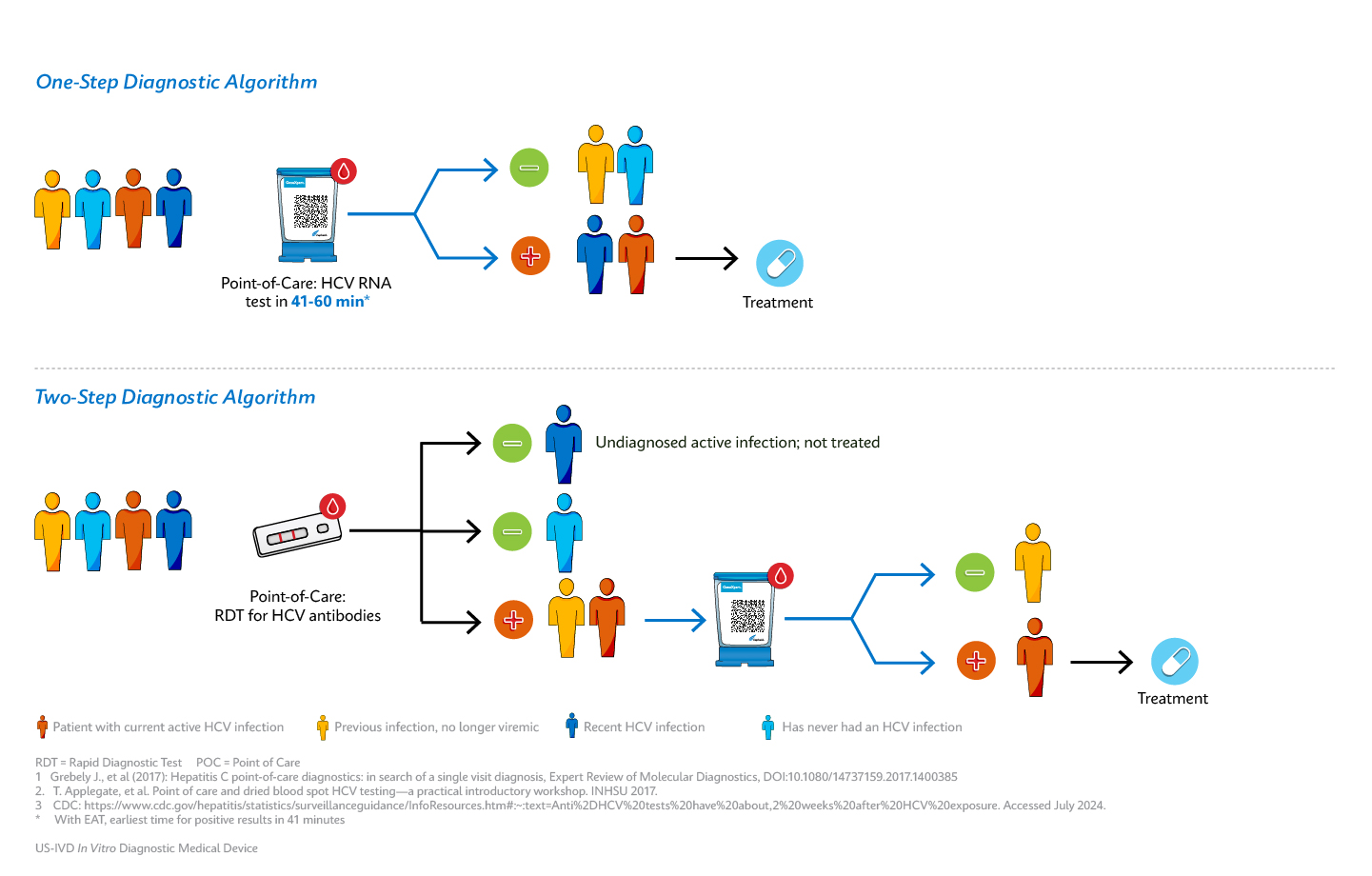
Hepatitis C Virus (HCV) testing in the U.S. is inadequate to achieve the goal of HCV elimination. The current diagnostic algorithm requires two testing steps – a screening test for HCV antibodies, followed by an RNA-based test to detect active infection. This algorithm is suboptimal and has various pain points, including a low screening rate due to a lack of adequate testing infrastructure, results that take days to weeks to be returned, patients lost to follow-up, ongoing transmission risk, and barriers related to health insurance.1
Testing for HCV antibodies alone is insufficient to initiate treatment, as it does not confirm the presence of active infection. Detectable levels of HCV antibodies significantly lag detectable HCV RNA, and antibody tests cannot detect acute HCV infections during the window before seroconversion.2
Additionally, there is a significant disparity in access to HCV testing, particularly among the populations that need it the most. Let's explore the challenges different population groups face, the importance of robust screening programs, and the role of innovative solutions in achieving HCV elimination.
Disparities in Access to HCV Testing
The general U.S. population's seroprevalence of HCV antibodies ranges from 0.3% to 6.8%.* This group typically has insurance coverage and is more likely to seek routine healthcare, leading to more effective HCV screening and follow-up.
However, there are specific populations that are at high risk for HCV infection, including people who inject drugs (PWID), American Indians/Alaska Native populations (AI/AN), and incarcerated individuals. In these populations, the seroprevalence of HCV antibodies can be as high as 18-88% in certain geographical areas.3
People who are at higher risk for HCV infection also face challenges in accessing routine health care in general. A testing algorithm that requires multiple visits before treatment can be initiated results in loss to follow-up and eligible patients remaining untreated. Settings that serve patients at risk for HCV infection (i.e.: syringe services programs, outreach programs, and carceral settings) often lack robust and regular screening programs.
In the U.S., there is a significant unmet need to expand HCV testing infrastructure to care settings that reach populations at high risk for HCV infection.
Key Learnings and Real-World Implementation
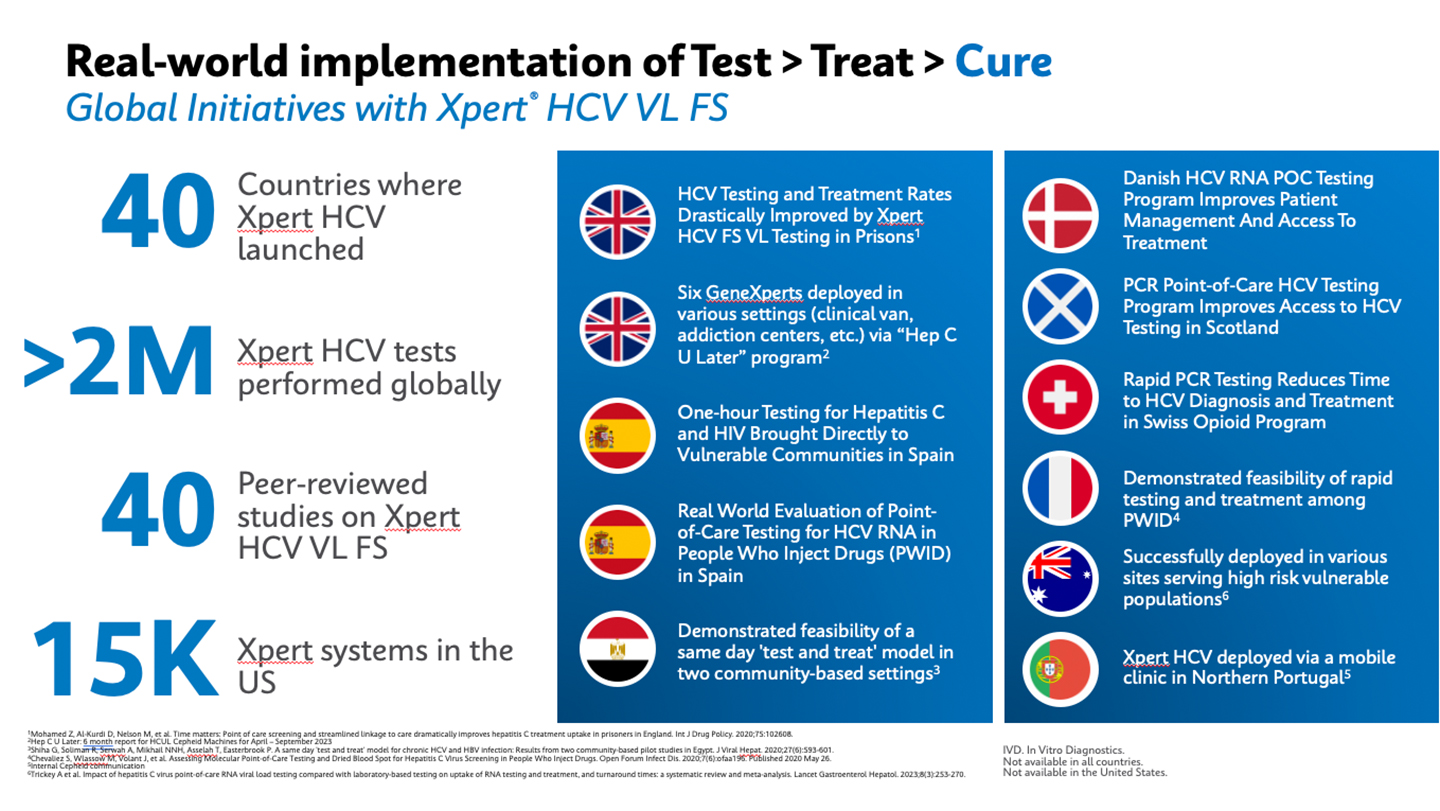
Over the past several years, Cepheid has gained valuable insights into promoting access to HCV testing via its PCR-based fingerstick test, Xpert® HCV VL FS.
Cepheid's experience launching this test in Europe, Africa, and Australia has successful HCV test and treat programs include having the instrument, the test, and the ability to prescribe treatment seamlessly. Additionally, offering pan-genotypic drugs on-site helps minimize the risk of loss to follow-up.
Phlebotomy, the process of drawing blood for testing, can be challenging in non-clinical settings. To overcome this, capillary blood collection has proven to be the most efficient method for accessing testing at non-clinical sites. Since the test received the CE-IVD mark in 2018, Xpert® HCV VL FS has been launched in 40 countries, serving global cure initiatives worldwide.
Point-of-care HCV Test and Treat Models
Studies have successfully demonstrated improved clinical outcomes associated with Xpert HCV VL FS testing at the point of care (POC). These outcomes include increased HCV diagnosis, reduced time to treatment initiation, increased treatment uptake, and reduced forward transmission.
Bringing the test to where patients are present has been a success story in Australia since July 2017, when one community used a mobile van to deliver testing.5 In the Kombi Clinic, a yellow 1975 Volkswagen van, a group of clinicians drive to meet patients at risk for HCV infection where they are and provide testing using Cepheid’s tests.

Source: https://www.kombiclinic.com/about-us
Using the Kombi Clinic, HCV RNA tests are performed in a mobile setting, and positive patients can start treatment within a week with a Kombi doctor or nurse practitioner. Qualitative data gathered from Kombi patients regarding the acceptability of POC testing was overwhelmingly positive.
Toward HCV Elimination
Cepheid's efforts align with global and national initiatives to accelerate HCV elimination. Achieving HCV elimination goals requires alignment of policies, guidelines, and payer/reimbursement systems, all driven by evidence-based approaches and centered on healthcare access and health equity. Cepheid’s recent launch of Xpert HCV, a U.S. FDA-cleared test for use at the POC (in CLIA-waived settings), supports the National HCV Elimination Plan proposed by the United States White House.
A key component of this plan is to identify more cases by expanding access to single-visit, rapid-result testing with immediate linkage to care and treatment.4 The test is intended for adult patients at risk and/or with signs and symptoms of HCV infection, with or without evidence of HCV antibodies.
The test can provide results in under an hour, enabling testing and treatment initiation in the same visit.
The Need for a Future Diagnostic Algorithm
To achieve HCV elimination, it is crucial to adopt a diagnostic algorithm that addresses the shortcomings of the current approach. This algorithm should focus on improving access to RNA-first diagnostic testing and ensuring linkage to care and treatment initiation. Same-day test and treat models of care are now possible in the U.S. With the recent FDA authorization of Xpert HCV in the U.S., we can now diagnose HCV with a single test that can be performed at the POC, and patients who are diagnosed with HCV can leave their visit with treatment in hand.
Innovative solutions and collaborations between healthcare organizations, government bodies, and regulatory agencies pave the way for improved access and better diagnostic solutions. By addressing the challenges and implementing evidence-based strategies, we can work towards a future where HCV elimination becomes a reality.
The information provided in this article is for educational purposes only and should not be considered as medical advice. Please consult a healthcare professional for personalized guidance on HCV testing and treatment.
IVD. In Vitro Diagnostic Medical Device. Not all tests are available in all countries.
References:
1. Yumi Sheehan, Evan B. Cunningham, Amanda Cochrane, Marianne Byrne, Tracey Brown, Colette McGrath, Lise Lafferty, Nicodemus Tedla, Gregory J. Dore, Andrew R. Lloyd, Jason Grebely, A ‘one-stop-shop’ point-of-care hepatitis C RNA testing intervention to enhance treatment uptake in a reception prison: The PIVOT study, Journal of Hepatology, Volume 79, Issue 3.
2. Diagnosis of Acute HCV Infection. Hepatitis C Online. Accessed May 16, 2024. https://www.hepatitisc.uw.edu/pdf/screening-diagnosis/acute-diagnosis/core-concept/all
3. AASLD, IDSA HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C: https://www.hcvguidelines.org/unique-populations/pwid#:~:text=Prevalence%20of%20HCV%20Among%20People,)%3B%20(Amon%2C%202008).
4. United States National Hepatitis C Elimination Initiative.
Accessed May 22, 2024.
5. Kombi Clinic. https://www.kombiclinic.com/about-us. Accessed July 3, 2024
Footnote:
*Estimates calculated internally based on IQVIA claims data for HCV testing.
Read Next
MORE







