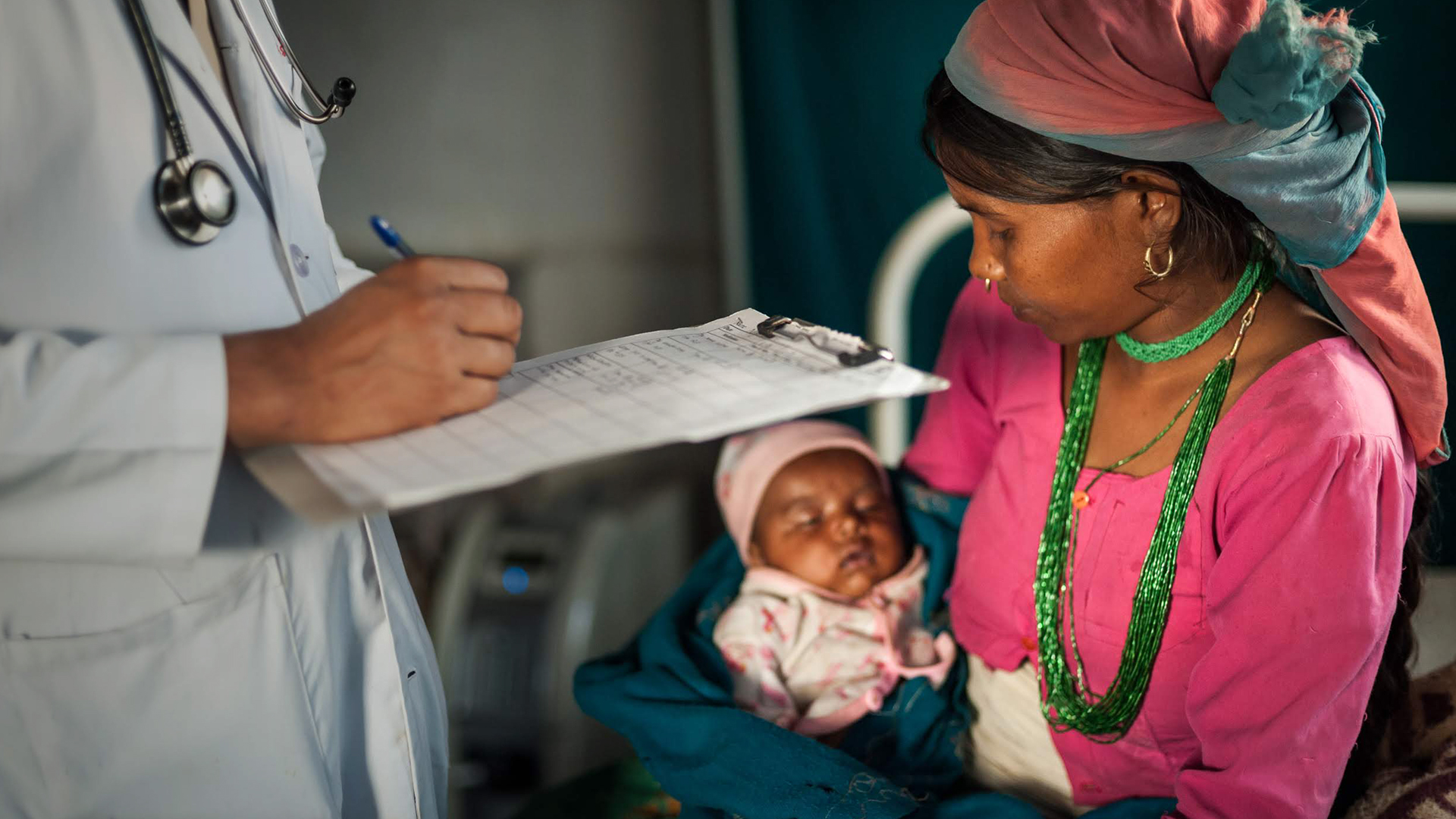
Enabling Global Health Solutions
With the increasing popularity of the GeneXpert® System, as well as the technology’s enormous potential to facilitate the diagnosis of multiple diseases in individual patients, Cepheid recognizes its responsibility in continuing critical test development and enabling platform access beyond the traditional laboratory.
A Global Need For Better Choices
Globally, these are mission-critical for Cepheid as a leading diagnostics company — because control of worldwide health threats may be hampered when testing is performed only in centralized labs, far from where patients live. Patients often travel long distances for diagnostic testing, or their samples must be transported — which may delay test results and impact patient care. Moreover, test results may get lost or never make it back to the patients.
Decentralization of testing facilities increases access and improves patient outcomes, as shown by recent studies from countries like Malawi1, where infants were tested for HIV both immediately at the point of care and by sending samples to a remote laboratory. More results were delivered to clinicians and more babies treated when tested locally.
The more than 10,000 GeneXpert Systems that have been installed in low and middle-income countries2, mainly for the diagnosis of active tuberculosis, can also be used to run tests for the nearly 30 CE-IVD3 Xpert tests now available.
Click here to download the Framework for GeneXpert Systems HIV and TB Integration

Outcomes That Speak For Themselves
Global health organizations, countries, donors and other stakeholders have been encouraging and supporting the more integrated, efficient use of diagnostics to work toward WHO targets for eliminating diseases such as HIV4, tuberculosis, HCV4 and cervical cancer.
"Unitaid has made significant investments in advancing integration in molecular diagnostics. Integrating care means more people healed, more lives saved, more efficient use of resources," Unitaid Executive Director Lelio Marmora said. "Now we need manufacturers to share in the responsibility of making sure these technologies work seamlessly in countries as part of sustainable systems."
"Integrating care means more people healed, more lives saved, more efficient use of resources. Now we need manufacturers to share in the responsibility of making sure these technologies work seamlessly in countries as part of sustainable systems."

HPV Testing in Cape Town – See How One Clinic is Making a difference for Women
There are many health challenges, particularly for women, in South Africa. Learn how a community-based clinic research project is making a difference.

ZDoggMD Discusses Tuberculosis with Cepheid CMO Dr. Dave Persing
Each day, nearly 4,500 people lose their lives to TB and close to 30,000 people fall ill. ZDoggMD and Dr. Persing discuss the global impact of a fast and accurate TB test that also detects drug resistance in the United States and globally.

Cepheid interviews Miss South Africa, Tamaryn Green
Miss SA gets behind the @StopTB campaign with the Cepheid SA team. #ItsTime to ensure access to rapid TB diagnostic testing for all #ItsTimetoEndTB #BreaktheStigma@TamarynGreen#BreaktheStigma

Tanzania Clinic & Cepheid GeneXpert HIV Testing
An incredible story of a study in rapid HIV testing on mothers and babies in Rural Tanzania.

HPV Testing in Cape Town – See How One Clinic is Making a difference for Women
There are many health challenges, particularly for women, in South Africa. Learn how a community-based clinic research project is making a difference.

ZDoggMD Discusses Tuberculosis with Cepheid CMO Dr. Dave Persing
Each day, nearly 4,500 people lose their lives to TB and close to 30,000 people fall ill. ZDoggMD and Dr. Persing discuss the global impact of a fast and accurate TB test that also detects drug resistance in the United States and globally.

Cepheid interviews Miss South Africa, Tamaryn Green
Miss SA gets behind the @StopTB campaign with the Cepheid SA team. #ItsTime to ensure access to rapid TB diagnostic testing for all #ItsTimetoEndTB #BreaktheStigma@TamarynGreen#BreaktheStigma

Tanzania Clinic & Cepheid GeneXpert HIV Testing
An incredible story of a study in rapid HIV testing on mothers and babies in Rural Tanzania.

Xpert® MTB/RIF Ultra

Xpert® HIV-1 Viral Load

Xpert® HIV-1 Qual

Xpert® HPV

Xpert® HCV Viral Load

Xpert® HBV Viral Load
1. Mwenda et al. Significantly improved antiretroviral therapy initiation rates after implementation of Point of Care Early Infant Diagnosis. ASLM 2016
2. HBDC Access Program covers 130 Low and Middle-income countries. Conditions can be found on the FIND website (https://www.finddx.org/pricing/genexpert/)
3. Some products not available in the US