5m Read
September 10, 2024
COMMUNITY AND GLOBAL HEALTH
Expert Perspective
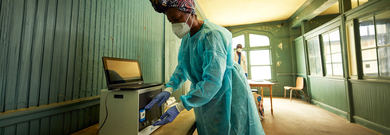
Diagnostics: A Key to Pandemic Preparedness in Resource-Limited Regions
Diagnostic tests are fundamental to successful outbreak control strategies. The COVID-19 pandemic revealed the need for strong, collaborative health systems worldwide, especially in resource-limited regions with unique needs. One estimate puts the likelihood of another COVID-19-scale pandemic by 2049 at 47%–57%1 which highlights the urgent need for planning and preparation. The World Health Organization’s Global Preparedness Monitoring Board (GPMB)2 and Preparedness and Resilience for Emerging Threats (PRET)3 initiative, among others, offer key steps to prepare for the next pandemic at global and national levels. Diagnostics has a key role in preparedness plans.
Watching & Connecting
Effective surveillance systems spot outbreaks early. The GPMB stresses the need for real-time data collection and analysis. This requires a network of connected systems that can quickly send data from remote areas to central databases. But smooth connectivity requires solutions to issues like data privacy and unstable internet in low and middle-income countries (LMICs).
In 2018, prior to the COVID-19 pandemic, Seth Berkley, the CEO of Gavi, the vaccine Alliance noted that early detection through diagnostic testing is essential to avoiding outbreaks: “Prevention is always the first line of defense, and nations must maintain vigilant surveillance—and yet, effective and affordable, quick and definitive diagnostics are absent in the countries where they are most needed.”4 In a prescient warning, Berkley continued, “This represents one of our most serious global health security blind spots.” Sadly, even after a recent global pandemic, broader access to effective diagnostics is still a need in many LMICs.
Strong & Flexible Labs
Labs must handle sudden increases in testing and work under unprecedented conditions. The old idea of always using lab instruments at full capacity and in program silos has changed. Now, it's important to be ready for sudden testing surges. Modern diagnostic tools are robust, can support program integration, and are quick to set up, which is crucial in emergencies.
During the COVID-19 pandemic, labs that were already leveraging robust testing systems for other diseases had the agility to deploy these systems for SARS-CoV-2 testing. A recent publication noted that “There is also a major crossover benefit from TB testing to COVID-19 and respiratory diseases. During 2020-2021 the high-performing countries used their TB diagnostic equipment, especially GeneXpert and other PCR diagnostic machines, to test simultaneously for TB and COVID-19.”5 This ability to pivot in the face of change highlights the benefit of a robust testing platform and the need to strengthen access and availability of such systems ahead of potential threats.
Additionally, the ability to run tests both in centralized lab environments and in decentralized community settings, closer to the point of need, adds an element of flexibility and patient access that could benefit health systems in LMICs.
Training the Workforce
Ongoing education of lab and healthcare workers is essential for pandemic readiness. A well-trained workforce can use diagnostic tools more effectively and respond sooner to health threats. Laboratory staff need to be sensitized to equipment and test results, while clinical staff need to create and/or update diagnostic and treatment algorithms to use test results to inform appropriate treatment.
The availability of easy-to-use tests and equipment can be a crucial factor in the face of workforce training. A simple user interface and tests with few manual steps shorten training time and can minimize the risk to laboratory workers, which is particularly important for pathogens that are easily transmittable.6 Additionally, closed testing systems, such as those employing single-use test cartridges or cassettes, can reduce the risk of cross-contamination, making testing safer and simpler for users.
While governments and non-governmental agencies may need to coordinate training at a national level during pandemic conditions, training provided by medical device manufacturers can have significant local reach. Cepheid, for example, offers free training programs in multiple languages as part of their Global Access Program for LMICs. These trainings, available in-person and online to laboratory staff, NGOs, and implementing partners, strengthen local healthcare capacity and ensure optimal use of Cepheid’s GeneXpert solutions.
Multipathogen Testing & Integration
Running multiple tests on one platform saves time and increases efficiency. This is especially important during outbreaks when quick results are needed. In its COVID-19 Response Mechanism Information Note,7 the Global Fund outlines recommendations for transitioning from COVID-19 response to resilient and sustainable systems for health, community system strengthening, and pandemic preparedness. The guidance notes that “a functional, integrated, tiered laboratory system with level-appropriate diagnostic testing is necessary” and encourages the use of multipathogen testing instruments in integrated laboratory diagnostic networks that use common laboratory instruments to support multiple testing streams.7
This multipathogen testing approach is detailed in the WHO’s Considerations for Adoption and Use of Multi-disease Testing Devices in Integrated Laboratory Networks.8 The WHO notes that the introduction of technologies that can test for multiple diseases “brings new opportunities for collaboration and integration, which can provide significant system efficiencies and cost savings, increase patient access, and ultimately improve quality of care.”
Such integration is not trivial, and requires coordinated planning by ministries of health, regulatory approval and possibly in-country validation, careful product and site selection, setting up integrated specimen referral systems, operating procedures, training, and management. Such multipathogen testing and integration are possible, however, and the WHO highlights a case study of the adoption and use of GeneXpert systems by TB and HIV programs.8
Global Access & Health System Resiliency
The World Bank's Pandemic Fund and the World Health Assembly’s Pandemic Accord emphasize international cooperation and offer financing to strengthen health system resiliency. Global donors, policymakers, governments, NGOs, and national and local health systems are helping change health initiatives to maximize impact.
Medical device manufacturers and other commercial entities are also making contributions toward the goal of strengthening health systems. Cepheid's Global Access Program began in 2011 with a vision of providing equitable access to revolutionary new molecular diagnostics. Today, the program reaches every corner of the globe impacted by deadly diseases like Tuberculosis, HIV, and Ebola. With over 20,000 instruments placed in low and middle-income countries, the program supports the worldwide access and response needed to manage outbreaks.
Diagnostics are a key component of pandemic preparedness. Integration of accessible and effective diagnostics into existing preparedness systems and plans, and the overall strengthening of diagnostic capacity in LMICs will help detect, respond to, and manage future outbreaks – ultimately saving lives.
References:
1. Smitham E, Glassman A. The Next Pandemic Could Come Soon and Be Deadlier. Center For Global Development Blog Post [Internet]. 2021 Aug 25 [cited 2024 Jul 10]; Available from: https://www.cgdev.org/blog/the-next-pandemic-could-come-soon-and-be-deadlier
2. A Fragile State of Preparedness: 2023 Report on the State of the World’s Preparedness. Global Preparedness Monitoring Board, Geneva: World Health Organization [Internet]. 2023 [cited 2024 Jul 10]. Available from: https://www.gpmb.org/docs/librariesprovider17/default-document-library/2023-report.pdf
3. Preparedness and Resilience for Emerging Threats Module 1: Planning for respiratory pathogen pandemics [Internet]. [cited 2024 Jul 10]. Available from: https://www.who.int/publications/i/item/9789240084674
4. Berkley S. Health security’s blind spot. Science. 2018 Mar 9;359(6380):1075.
5. Soe-Lin S, Bowen W, Hecht R. To Prepare For The Next Pandemic, We Must Spend Now On TB And Other Major Diseases [Internet]. Health Affairs Forefront. 2024 [cited 2024 Jul 10]. Available from: https://www.healthaffairs.org/content/forefront/prepare-next-pandemic-we-must-spend-now-tb-and-other-major-diseases
6. Kelly-Cirino CD, Nkengasong J, Kettler H, Tongio I, Gay-Andrieu F, Escadafal C, et al. Importance of diagnostics in epidemic and pandemic preparedness. BMJ Glob Health. 2019 Jan 29;4(Suppl 2):e001179.
7. COVID-19 Response Mechanism Information Note. Transition from the COVID-19 Response to Resilient and Sustainable Systems for Health, Community System Strengthening and Pandemic Preparedness [Internet]. 2021 [cited 2024 Jul 10]. Available from: https://www.theglobalfund.org/media/10749/covid19_c19rm-technical_informationnote_en.pdf
8. Considerations for adoption and use of multidisease testing devices in integrated laboratory networks: information note [Internet]. [cited 2024 Jul 10]. Available from: https://iris.who.int/handle/10665/255693
Read Next
MORE