The GeneXpert® system from Cepheid enables standardized, central-lab quality molecular diagnostic testing at the point-of-care to:
• Accelerate clinical trial enrollment by brining the test to trial sites
• Reach diverse populations through a point-of-need test-and-treat program
• Improve accessibility of companion and complementary diagnostics
Cepheid has Partnered with Pharma to Support Clinical Trials Worldwide for over a decade(1)
Cepheid's Statistics For Clinical Trial and Test and Treat Programs Since 2020 Include:*

Why do Pharma Sponsors Choose Cepheid?
• Accurate results sponsors and trial sites can rely on.
• Speed and efficiency with on-demand answers WHEN they are needed most to accelerate patient recruitment and enrollment
• Simplicity - a solution that is easy to learn, adopt, and operate across a variety of settings and users
• A flexible delivery platform - the GeneXpert system and Xpert tests cartridges can deliever results WHERE they are needed, from the central lab to remote trial sites to mobile testing
• Quality of text design - a proven track record of delivering quality across a broad menu of test products. Experience matters.
• Global operational and regulatory expertise supports patient enrollment worldwide
The Preferred Simple, and Real-time Diagnostic Solution for Clinical Trials
The GeneXpert system is a point-of-care molecular diagnostic solution, automating the complex, manual steps of polymerase chain reaction (PCR), while combining fast turnaround times with simple workflows for real-time PCR results.
Testing as simple as 1, 2, 3
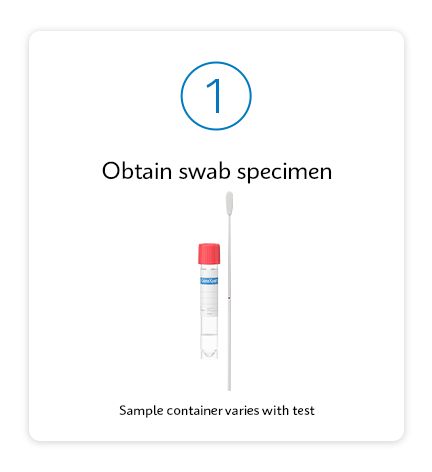
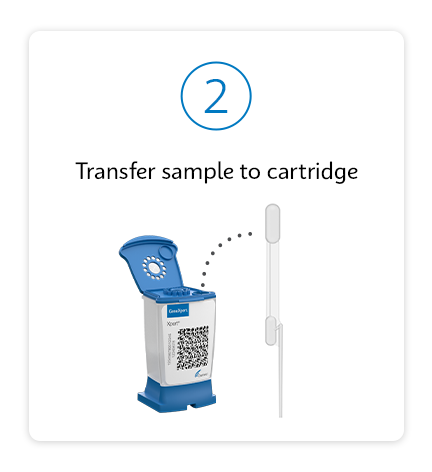

Improve Patient Outcomes with a Test-and-Treat Program
Xpert® tests provide medically actionable information for patients to receive same-day treatment

The Xpert Cartridge
Sample extraction, PCR amplification, and detection are all carried out within this lab in a cartridgeTM
All Xpert tests can run on any GeneXpert system, providing random access to 2-80 tests simultaneously–no need for batching.

Comprehensive PCR Menu
The GeneXpert® family of systems can run any of Cepheid's 34 CE-IVD and 22 US-IVD tests to provide accurate, high-quality, real-time PCR results.
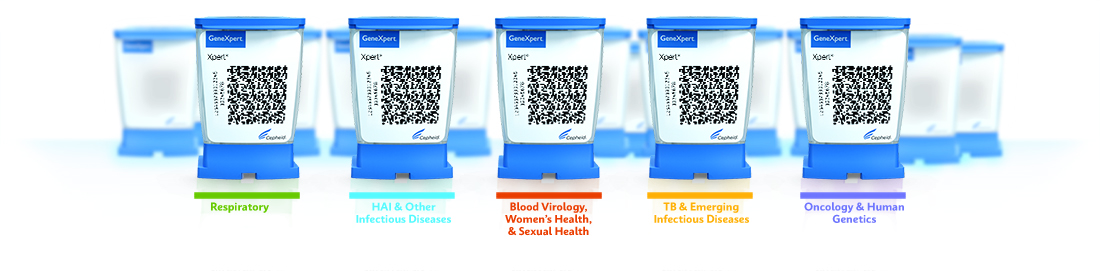
GeneXpert Family of Systems
Available in two, four, 16, 48, or 80-module configurations, the GeneXpert platform is
both standardized and scalable across the clinical trial continuum — from central labs to remote trial sites to mobile testing.


Journey Inside the Cepheid GeneXpert® Cartridge - 3D Animation
The cornerstone of the GeneXpert testing process is Cepheid's patented, self-contained, single use cartridge. Sample extraction, amplification and detection are all carried out within this self-contained "laboratory in a cartridge".

Meet our Game-Changing Platform
With over 36,000 installed systems worldwide, the GeneXpert System is the most popular sample-to-answer PCR testing system. See how it’s helping clinicians everywhere make a more positive impact.

How a Cepheid Molecular Test is Made — with Dr. Fred Tenover, Vice President, Scientific Affairs
Cepheid's Dr. Fred Tenover walks us through the process of developing, manufacturing and distributing a molecular diagnostic test for infectious diseases like MRSA, COVID-19, Flu, TB and others. There's a lot to this complex task, and Dr. Tenover is here to help us make sense of it all.

Journey Inside the Cepheid GeneXpert® Cartridge - 3D Animation
The cornerstone of the GeneXpert testing process is Cepheid's patented, self-contained, single use cartridge. Sample extraction, amplification and detection are all carried out within this self-contained "laboratory in a cartridge".

Meet our Game-Changing Platform
With over 36,000 installed systems worldwide, the GeneXpert System is the most popular sample-to-answer PCR testing system. See how it’s helping clinicians everywhere make a more positive impact.

How a Cepheid Molecular Test is Made — with Dr. Fred Tenover, Vice President, Scientific Affairs
Cepheid's Dr. Fred Tenover walks us through the process of developing, manufacturing and distributing a molecular diagnostic test for infectious diseases like MRSA, COVID-19, Flu, TB and others. There's a lot to this complex task, and Dr. Tenover is here to help us make sense of it all.
US-IVD. In Vitro Diagnostic Medical Device.
CE-IVD. In Vitro Diagnostic Medical Device. May not be available in all countries.
*data retrieved from internal Cepheid reports.
1. PR Newswire. Cepheid Collaborates With Pharmaceutical Leader on Chronic Myelogenous Leukemia. Accessed Oct. 12, 2022. http://cepheid.mediaroom.com/2010-10-07-Cepheid-Collaborates-With-Pharmaceutical-Leader-on-Chronic-Myelogenous-Leukemia





.png)